An acetic acid solution 10 ml saturated with hbr sat.
Methyl vinyl hbr.
Au orr ewing andrew.
N2 elimination of hbr from uv photoexcited vinyl bromides can occur through both 3 centre and 4 centre transition states tss.
After the addition was complete the temperature was raised to 55 c for 30 min.
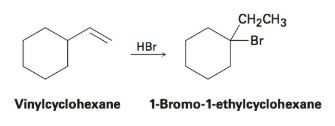
In the following reaction of the addition of hbr to 1 methyl 1 vinylcyclopentane 1 one of the major products formed is 1 bromo 1 isopropylcyclopentane 2.
The three vinyl bromides chosen for study have methyl substituents tha.
It is soluble in water and polar organic solvents.
Au hornung balazs.
T1 direct comparison of 3 centre and 4 centre hbr elimination pathways in methyl substituted vinyl bromides.
Methyl vinyl ketone mvk iupac name.
However under anhydrous conditions at room temperature it undergoes many addition reactions at the double bond electrophilic addition reaction more favourable.
If methanol reacting as water would and if this reaction follows a typical mechanism of electrophilic addition what would be the expected product.
Vinyl ether 50 1 20 g 0 007 mol was dissolved in acetic acid 5 ml and warmed to 40 c.
The competition between these pathways is examined using velocity map imaging of hbr v 0 2 j photofragments.
Of hbr v 0 2 j photofragments.
Au pandit shubhrangshu.
It is hydrolysed rapidly by dilute acids at room temperature to give methanol and aldehyde.
Methyl vinyl ether is a very reactive gas.
Butenone is the organic compound with the formula ch 3 c o ch ch 2 it is a reactive compound classified as an enone in fact the simplest example thereof it is a colorless flammable highly toxic liquid with a pungent odor.
The kinetic energy distributions extracted from velocity map images of hbr from 193 nm photolysis of the three vinyl.
Elimination of hbr from uv photoexcited vinyl bromides can occur through both 3 centre and 4 centre transition states tss.
The mechanism for this reaction involves the formation of intermediate a which is converted into intermediate b and finally product 2 is formed.
4 methyl 2 pentyne c6h10 cid 140789 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more.